|
Radioactive pollution results from harmful radiations emitted from radioactive substances. The sources of radiation can be natural (82%) and anthropogenic (18%). Radioactivity Radioactivity is not an alien concept. We are naturally exposed to radiation from radioactive minerals within the earth’s crust as well as from outside the earth, and from outer space (cosmic rays). Radioisotopes like carbon 14, potassium 40, radon 222, radium 224, uranium 235, and uranium 238 occur abundantly in rock, soil, and water. We breathe radiative gases, and we have radioactive substances in our bodies. We are exposed to various low levels of radiation from man-made sources in our homes, schools, and offices. The most common of these exposures is medical-X-rays; radiation used to diagnose diseases. On average, a person is exposed to about 350 millirems (US unit of effective dose) of nuclear radiation per year in the US.  Image Source: US EPA Image Source: US EPA Fallout of radioactive materials from nuclear explosive testing, coal, and nuclear power plants are also sources of radioactive pollution. Though it comprises less than 0.5% of the total radiation human dose, radioisotopes from nuclear testing linger in the environment for over 100 years. Other responsible factors for exposure to high doses of radiation can be geographical location, occupation (mining), and personal lifestyle (smoking). So, let’s make ourselves theoretically familiar with the term radioactivity. It is simply the disintegration of atoms. The atom can be defined by the number of neutrons in the nucleus, while the protons balance electrons. Some elements are unstable in their natural forms. Their nuclei disintegrate and release energy in the form of alpha, beta, and gamma radiations in the process of radioactivity. Seventy radioactive elements in our periodic table decay from one isotope to another until they reach a stable one. The international unit for measuring radioactive decay is becquerels or Bq (one becquerel=one disintegration per second). The time taken by half radionuclides to decay is called the half-life, and it is very specific for each element. For example, the half-life of Iodine is eight days and that of Uranium is 4.5 billion years. Types of radioactive radiations Nuclear radiations are generally ionizing. They may cause ionization of the atoms and molecules of the medium through which they pass, converting them into ions, hence also known as ionizing radiations. Here’s a quick flashback to the most common ionizing radioactive radiations you must have read about in your high school:
Causes of Radioactive Pollution Radioactive pollution occurs when radioactive materials or waste is exposed to the environment, either by accident or human activities like nuclear weapon tests, nuclear power plants, and nuclear waste. 1- Radioisotopes from weapons of mass destruction and nuclear weapon testing The effects of the use of radioactive substances as weapons of mass destruction like missiles and atomic bombs on Hiroshima and Nagasaki can be seen to date. Though it promptly ended World War II in 1945, children are still born with mental retardation, autism, and birth abnormalities. Nuclear weapon testing releases a great chunk of radioactive materials into the environment. The most common of these radioactive substances are Iodine-131, Cesium-137, and Strontium-90. All of these pose a great risk to human health. Iodine-131, for example, is the most toxic among these. Although it has a half-life of 8 days, high doses can lead to thyroid cancer. Cesium-137 has a half-life of 30 years. It is chemically identical to potassium and is widely distributed throughout our bodies. Strontium-90 with a half-life of 38 years is often mistaken for calcium and gets absorbed in our bones. Once deposited in our bodies, their slow decay can cause severe biological damage, including cancer. 2- Nuclear power plants Another major source of nuclear contamination is nuclear power plants. It is touted as the most potent source of energy, but it is fraught with the risk of catastrophic nuclear accidents and unmanageable amounts of nuclear waste. A nuclear reactor, however, exposes individuals living on the fence line of the power plants to no more than 10 millirems of radiation per year. This radiation exposure is negligible when compared to 350 millirems of radiation from all other sources, mainly natural. In the US, coal and oil power plants contribute larger proportions of airborne radioactive substances than nuclear power plants. Albeit, we cannot ignore the catastrophes bring down upon us by nuclear accidents. (I’ll get back to this later) 3- Nuclear waste Nuclear waste is the third major source of radioactive pollution worldwide. Highly radioactive or high-level waste comes either from nuclear power plants, weapon production factories, or nuclear waste recycling facilities worldwide. According to one report, more than a quarter-million metric tons of highly radioactive waste is stored in dry casks near these stations. Over 90,000 metric tons of radioactive waste are in temporary storage containers in the U.S. alone, waiting for its permanent disposal. What’s more alarming is that these aging containers have already begun to leak. 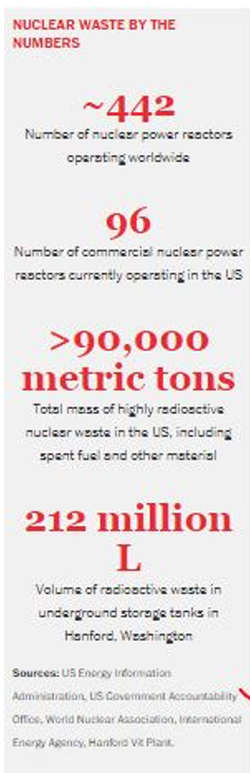 Image Source: CEN Image Source: CEN Other sources include mining operations, the use of radioisotopes in smoke detectors, and radiotherapy. Unlike conventional pollution, radioactive pollution can’t be detoxified or controlled unless the radioactive material reaches a stable form. Hence, it must be handled carefully and isolated from the environment until its radiation decreases to a safe level. Worst nuclear accidents in the history Of all the human-induced environmental disasters, nuclear disasters can cause the greatest damage. The worst of these disasters are Fukushima Daiichi-2011, Three Miles Island-1978, and The Chernobyl Disaster-1986. Nuclear accidents typically center on boiling nuclear reactors. Nuclear radiations from these disasters pose significant acute and chronic risks for longer periods, depending on their half-lives. These incidents and many others led to the prohibition of the use of nuclear power plants for electricity generation. Research is underway to put in place the best environmental safety practices. One of the most serious nuclear incidents in U.S. history is Three Miles Island (1979) in Pennsylvania, which resulted from the partial meltdown of reactor 2. Although this incident didn’t cause any human fatalities, people living within 80 km of the power plant were exposed to small amounts of radiation. Most of this comprised Iodine-131; short-lived yet lethal. According to one study, it could have increased the risk of thyroid cancer. The incident, however, led to psychological stress, mass evacuation, and distrust in the nuclear industry. The Chernobyl Disaster, Ukraine (1986) is the worst nuclear reactor accident to date. Lack of awareness of safety procedures led to a sudden power surge during the maintenance test of a reactor system, resulting in an explosion and destruction of Unit 4. More than 50 million Curies of radiation were released and spread across the western Soviet Union and Europe. Swedish radiation monitoring stations first reported abnormal (40 times higher than normal) radiation levels. The Chernobyl disaster led to the immediate death of 31 workers, while over 500 suffered from radiation burns and sickness. It resulted in the evacuation of more than 300,000 people from the plant’s vicinity. In terms of chronic effects, 10,000 people died from cancer and radiation-induced diseases in the most contaminated region, Belarus, Russia. The plant was permanently shut down in 2000. On March 11, 2011 earthquake-tsunami induced Fukushima Daiichi nuclear plant disaster, Japan resulted from overheating and melting of the reactor cores and ultimately hydrogen explosions. Although it didn’t cause any direct fatalities, the forced evacuation of 110,000 people in response to contamination of a wide area led to 1,600 deaths. Seems like people were better off staying put than evacuated. Health effects of radioactive pollution Even though we have small amounts of radioactive substances in our bodies, high doses of internal radioactive contamination, by any means, can affect our most sensitive (highly dividing) organs and tissues like skin, gonads, spleen, bone marrow, etc. Free radicals from ionizing radiation can disrupt the chemical makeup of important biomolecules such as DNA. High levels can either break DNA strands or lead to mutation in base pairs. Fortunately, our cells are highly efficient at repairing any damage. However, if the damage from radiation is not repaired properly, a cell may die or ultimately become cancerous. The display of effects depends on the nature of exposure (internal or external), time of exposure, the intensity of radiation, and the penetration power of the radiation. Exposure to high levels of radiation over a short duration can result in acute health effects like skin burns and radiation sickness (aka radiation syndrome) as observed in the Chernobyl Disaster. Exposure to low levels of environmental or background radiation is also a contributor to cancer risks. Control of radioactive pollution Radioactive pollution can be avoided in many ways:
References International Atomic Energy Association (IAEA) Radiation in Everyday Life Radioactive Pollution Disasters: Nuclear Accidents History’s worst Nuclear Disasters Radioactive Pollution | Radiation | Types, Contamination & Prevention Meltdowns, waste, and war: Here are the real risks of Nuclear A Brief History of Nuclear Accidents Worldwide AuthorNida Riaz is a freelance blogger based in Pakistan. She started writing about her passion for the environment when the world came to a stop in early 2020.
0 Comments
Leave a Reply. |
|
|
(833) CMS-LINE
(833) 267-5463 PO Box 13477 Mill Creek, Wa, 98082 © Conservation Made Simple. All rights reserved.
501(c)(3) Non-Profit, Tax ID#: 82-1646340 Copyright © 2021 Conservation Made Simple |





